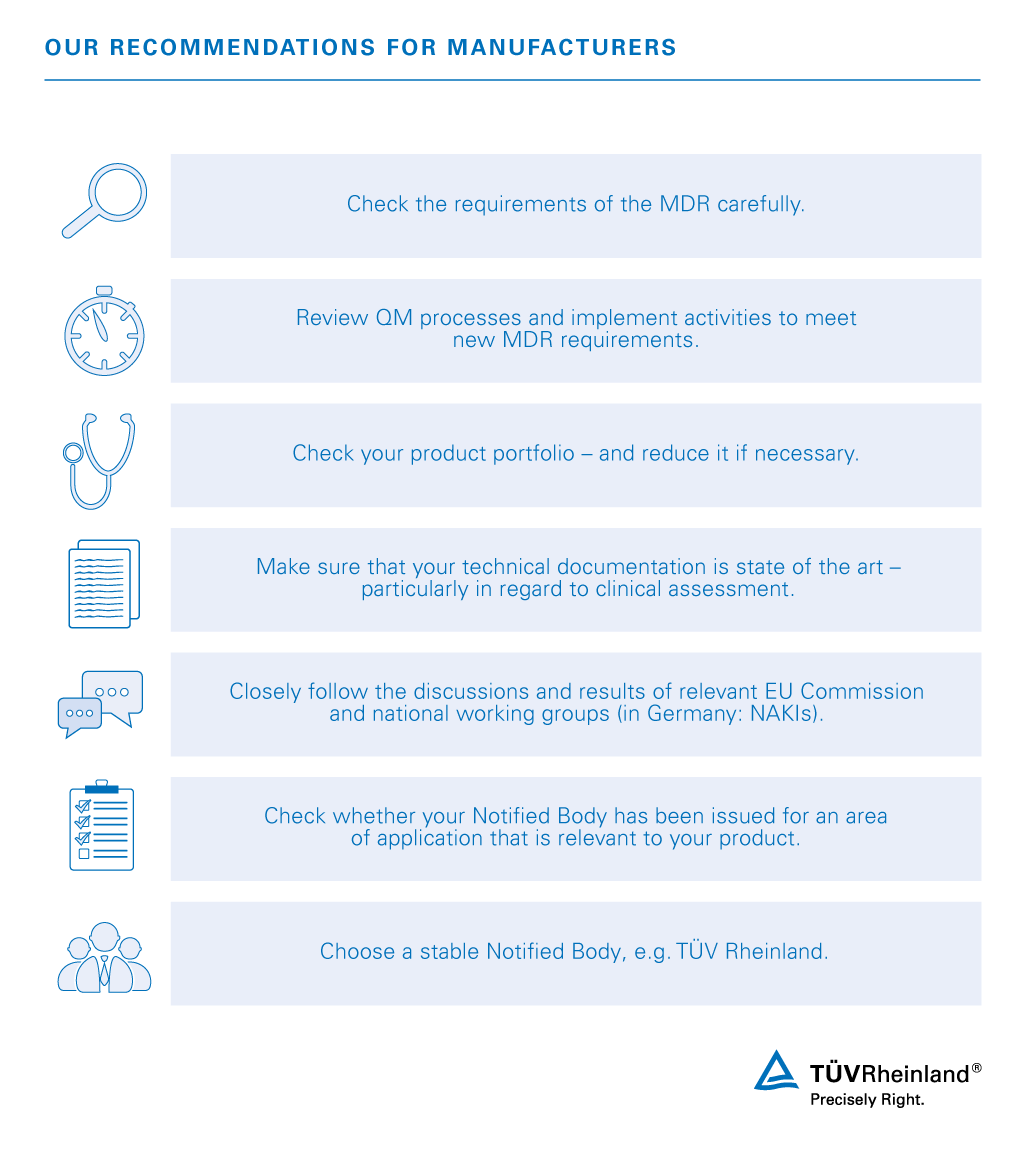
MDR: 26 Notified Bodies on NANDO & Swiss economic operator's requirements updated! · MDlaw – Information platform on European medical device regulations

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
Availability and capacity of notified bodies to carry out conformity assessments for COVID-19 related medical devices and in vit

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog